QA Review tab |

|

|
|
QA Review tab |

|

|
Usage:
Third tab is QA Review. Th is page is designed for Quality Assurance purposes.
Navigation: Customer Complaint Manager form > Open an existing complaint > QA Review
(Complaint Manager in the Main menu or open the Customer form > Select a Customer > navigate to Customer toolbar menu > Customer Complaints) .
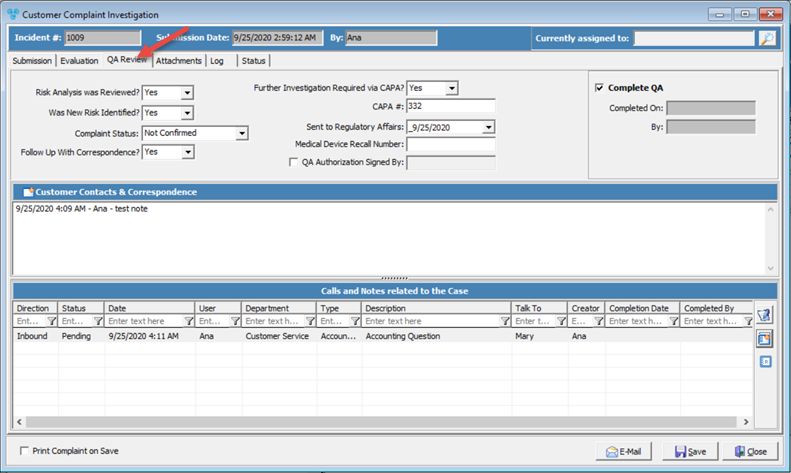
Complaint Investigation form with QA Review tab
What are the options on this form:
1. A couple of options that require Yes or No answers, such as: Reviewed Risk Analysis, New Risk Identified or Follow up with Correspondence.
2.Complaint Status - Select between 'Confirmed' or 'Not Confirmed'.
3. CAPA - Corrective Action and Preventive Action. Corrective and Preventive Action (CAPA) is a concept within Good Manufacturing Practice (GMP). CAPA focuses on the systematic investigation of discrepancies (failures and/or deviations) in an attempt to prevent their recurrence. To ensure that corrective and preventive actions are effective, the systematic investigation of the failure incidence is crucial in identifying the corrective and preventive actions undertaken. CAPA is part of the overall quality management system (QMS).
4. Sent to Regulatory Affairs - Enter date.
5.Medical Device Recall Number - Enter number if applicable.
6.QA Authorizing Signature - Check this after any necessary QA tasks have been reviewed and authorized.
7.Complete QA - The user who completed any necessary QA tasks should check this field. Once checked, the 'Completed On' and 'By' fields will be automatically filled in after save.
8. Customer Contacts and Correspondence - a note area to record the correspondence with the customers. To add Notes click on ![]() , type the info and click OK.
, type the info and click OK.
See also: